Rapid Diagnostics ML Limited have developed a saliva sample Real-Time PCR Point of Care test for the accurate detection of...
RAPID MINILAB
Realising the next generation of PCR diagnostics – The Rapid MiniLab™
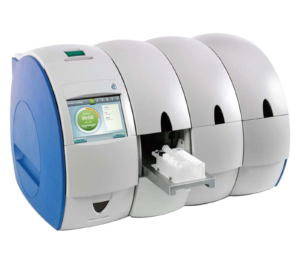
The Rapid MiniLab™ is the world’s 1st Point-of-Care (POC) Highly Multiplex PCR platform for both laboratory and decentralised POC diagnostic locations. Designed to comply with FDA CLIA Waiver guidelines, the system is easy to use with a simple cartridge delivering raw sample to result and a scalable architecture for any POC location. Results can be delivered in under 60 minutes providing accurate fast diagnosis and targeted treatment. Operators of the Rapid MiniLab™ system do not require specialist training allowing the system to be used in a broad range of settings in both developed and emerging healthcare countries.
Proprietary technology providing the next generation of integrated and automated real-time PCR-based diagnostics.
The Rapid MiniLab™ provides fully integrated and automated raw sample to result, real-time PCR diagnostic testing for laboratories, hospitals, clinics, physician surgeries and other decentralised POC locations.
Rapid’s simple-to-use products are specifically designed to deliver simple on-site results to clinicians and POC users.
The Rapid MiniLab™ is based on unique and proprietary technologies that are underpinned by its broad Intellectual Property portfolio of patents, trade marks and design rights.
Rapid was founded in 2017 and has its headquarters at Didcot, Oxfordshire, UK where it develops and manufactures the MiniLab and cartridges in a central modern facility. The Company is a private company registered in the UK.